
Thermal physics explores the relationships between heat, work, and energy, forming the cornerstone of modern physics. Kittel and Kroemer’s Thermal Physics provides a comprehensive framework for understanding these principles, bridging theoretical concepts with practical applications across diverse scientific fields.
1.1 Overview of Thermal Physics
Thermal physics is the study of heat, energy, and their interactions with matter. It encompasses the behavior of systems in thermodynamic equilibrium and their transitions. Kittel and Kroemer’s Thermal Physics introduces foundational concepts, including temperature, entropy, and energy distribution. The field bridges classical thermodynamics with statistical mechanics, exploring how macroscopic properties emerge from microscopic interactions. Understanding thermal physics is essential for analyzing phase transitions, energy conversion, and material properties, making it a cornerstone of modern scientific inquiry and technological advancement.
1.2 Importance of Thermal Physics in Modern Science
Thermal physics is fundamental to understanding energy interactions and material behavior. It underpins advancements in energy technologies, materials science, and biological systems. Kittel and Kroemer’s Thermal Physics highlights its relevance in explaining phase transitions, heat transfer, and thermodynamic processes. The field is crucial for developing sustainable energy solutions, optimizing industrial processes, and understanding complex biological systems. Its principles also apply to environmental science, aiding in climate modeling and resource management. This versatility makes thermal physics indispensable in addressing modern scientific and technological challenges.
1.3 Brief History of Thermal Physics
Thermal physics traces its origins to the 17th and 18th centuries, with foundational work by Boyle, Mariotte, and others on gas laws. The 19th century saw breakthroughs with the kinetic theory of gases by Maxwell and Boltzmann, and the formulation of the laws of thermodynamics by Clausius, Kelvin, and Planck. Einstein’s contributions to the quantum nature of light further advanced the field. The 20th century brought statistical mechanics and the work of Gibbs, laying the groundwork for modern thermal physics, as detailed in Kittel and Kroemer’s seminal textbook.
Key Concepts in Kittel and Kroemer’s Thermal Physics
Kittel and Kroemer’s Thermal Physics introduces fundamental concepts like temperature, thermodynamic equilibrium, and the laws of thermodynamics. It explores statistical mechanics, thermodynamic potentials, and phase transitions, providing a robust framework for understanding energy and its transformations in various systems.
2.1 Temperature and Thermodynamic Equilibrium
Temperature is a measure of the thermal energy of particles in a system, determining whether heat will flow between objects in thermal contact. Thermodynamic equilibrium occurs when a system’s properties, such as temperature and pressure, remain stable over time. Kittel and Kroemer’s text emphasizes the Zeroth Law of Thermodynamics, which introduces the concept of temperature and enables the creation of thermometers to measure it. This foundational concept is crucial for understanding energy distribution and system interactions in thermal physics.
2.2 Laws of Thermodynamics
The laws of thermodynamics govern energy interactions and transformations. The First Law, or energy conservation, states that energy cannot be created or destroyed, only converted. The Second Law introduces entropy, a measure of disorder, and establishes the direction of natural processes. Kittel and Kroemer’s text meticulously explains these principles, providing a robust foundation for understanding heat, work, and energy exchange. These laws are essential for analyzing systems in equilibrium and predicting the feasibility of physical processes in thermal physics.
2.3 The Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics establishes the concept of thermal equilibrium, enabling the definition of temperature. It states that if two systems are in thermal equilibrium with a third system, they are also in equilibrium with each other. This law is fundamental, as it allows the measurement and comparison of temperatures across different systems. Kittel and Kroemer’s text elaborates on this principle, illustrating its role in forming the basis of thermodynamic systems and interactions, ensuring consistency in energy exchange analyses.

Statistical Mechanics Foundations
Statistical mechanics links thermodynamics to microscopic properties, using probability to describe system behaviors. Kittel and Kroemer’s text provides a robust foundation, blending classical and quantum approaches effectively.
3.1 Classical Statistical Mechanics
Classical statistical mechanics provides a framework to understand macroscopic properties from microscopic behaviors. It relies on concepts like Maxwell-Boltzmann statistics and the idea of ensembles. Kittel and Kroemer explain how classical methods describe systems in thermal equilibrium, emphasizing the role of probability distributions. Recent studies, such as stochastic models of airway smooth muscle, illustrate the application of classical principles in complex biological systems, aligning with the foundational theories presented in the textbook.
3.2 Quantum Statistical Mechanics
Quantum statistical mechanics extends classical methods to systems governed by quantum mechanics. It introduces concepts like Fermi-Dirac and Bose-Einstein statistics, essential for understanding particles at microscopic scales. Kittel and Kroemer delve into quantum distributions and their applications in solids and gases. Recent research, such as studying liquid water through band theory, highlights how quantum principles like Fermi level shifts in RedOx processes explain material properties, aligning with the textbook’s foundational explanations of quantum systems and their thermodynamic behaviors.

3.3 The Role of Probability in Thermal Physics
Probability is central to thermal physics, enabling the analysis of systems with inherent uncertainty. Stochastic models, like the Ising-type antimagentic interactions and percolation processes, illustrate how probabilistic approaches describe phase transitions and collective behaviors. Kittel and Kroemer emphasize the statistical nature of thermodynamic systems, linking probability to entropy and equilibrium. This framework is vital for understanding complex phenomena, such as airway hyperresponsiveness, where probabilistic models explain tonic and phasic contractions, showcasing the broad applicability of thermal physics principles.

Thermodynamic Potentials
Thermodynamic potentials, such as internal energy and entropy, are crucial in understanding system equilibrium and energy transformations. They relate to variables like temperature and pressure, fundamental in Kittel and Kroemer’s analysis.
4.1 Internal Energy and Entropy
Internal energy represents the total energy of a system, including kinetic and potential energies of particles. Entropy, a measure of disorder, is central to the second law of thermodynamics. In Kittel and Kroemer’s Thermal Physics, internal energy and entropy are linked through thermodynamic processes, with entropy changes (ΔS) defined by reversible heat transfer divided by temperature (T). The third law of thermodynamics further establishes that entropy approaches a minimum value as T approaches absolute zero, providing a foundational framework for understanding energy transformations and system equilibrium.
4.2 Helmholtz Free Energy
Helmholtz free energy (F) is a thermodynamic potential defined as F = U ─ TS, where U is internal energy, T is temperature, and S is entropy. It is minimized at equilibrium for systems at constant temperature and volume. In Kittel and Kroemer’s Thermal Physics, Helmholtz free energy is explored as a tool to analyze phase transitions and spontaneous processes. It provides insight into the stability of thermodynamic systems and is crucial for understanding energy transformations under constrained conditions, making it a foundational concept in modern thermal physics studies.
4.3 Gibbs Free Energy
Gibbs free energy (G) is a thermodynamic potential defined as G = H ─ TS, where H is enthalpy, T is temperature, and S is entropy. It is particularly useful for systems at constant pressure and temperature. In Kittel and Kroemer’s Thermal Physics, Gibbs free energy is discussed as a key indicator of spontaneity and equilibrium in chemical reactions. A negative change in Gibbs free energy (ΔG) signifies a spontaneous process. It is widely applied in electrochemical systems and phase transitions, providing critical insights into energy transformations and system stability under real-world conditions.

Phase Transitions and Critical Phenomena
Phase transitions and critical phenomena are fundamental in thermal physics, describing how systems change states and behave near critical points, as detailed in Kittel and Kroemer’s work.
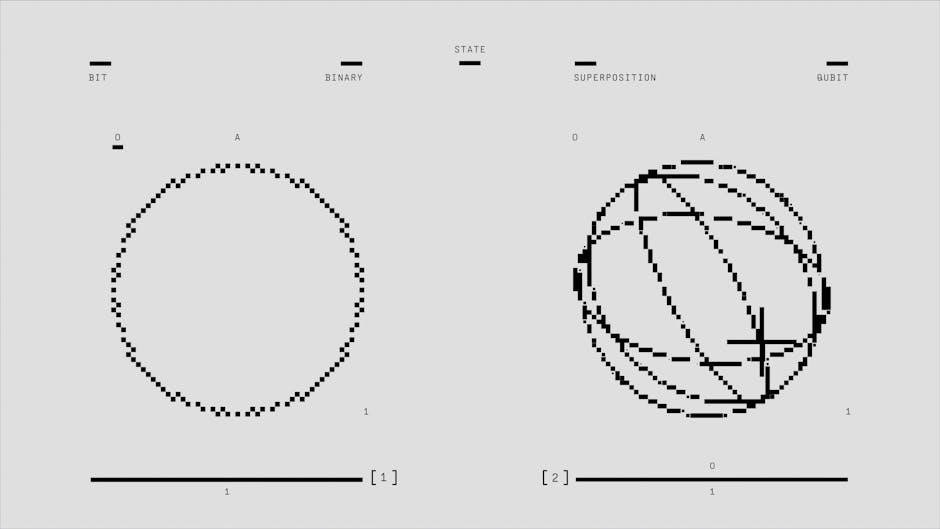
5.1 Types of Phase Transitions
Phase transitions are classified into first-order, second-order, and continuous transitions. First-order transitions involve latent heat, like melting ice, with discontinuities in thermodynamic properties. Second-order transitions exhibit continuous changes but show discontinuities in derivatives of free energy, such as the ferromagnetic phase transition. Continuous transitions are smooth without discontinuities. These classifications help understand material behavior under varying conditions, as discussed in Kittel and Kroemer’s analysis of thermodynamic systems and their responses to external parameters.
5.2 Critical Exponents and Universality
Critical exponents describe the universal behavior of physical systems near phase transitions. These exponents are independent of material details, reflecting underlying universality principles. For example, systems like magnets and fluids exhibit identical critical behavior near their phase transitions. Kittel and Kroemer emphasize how these exponents are determined by the system’s dimensionality and symmetry, rather than specific interactions. This universality simplifies understanding complex phenomena, as captured in their analysis of the Ising model and its applications to diverse phase transitions, including percolation processes and magnetic ordering.
5.3 Applications of Phase Transition Theory
Phase transition theory has broad applications in materials science, biology, and environmental systems. For instance, understanding liquid water’s behavior under varying RedOx potentials, as studied in band theory, reveals insights into chemical and biological processes. Additionally, phase transitions are crucial in modeling airway hyperresponsiveness, where stochastic models of smooth muscle contraction exhibit universal behaviors. Kittel and Kroemer’s textbook highlights how these concepts apply to real-world phenomena, from material design to biological systems, demonstrating the versatility of thermal physics in solving complex problems across disciplines.
Applications of Thermal Physics
Thermal physics applies to materials science, biology, and environmental systems, aiding in understanding phase transitions, energy efficiency, and biological processes, as highlighted in Kittel and Kroemer’s work.
6.1 Solid-State Physics and Materials Science
Thermal physics is crucial in solid-state physics, explaining phenomena like specific heat, thermal conductivity, and phase transitions in materials. Kittel and Kroemer’s text provides foundational theories for understanding these properties, enabling advancements in semiconductor design and nanomaterials. The principles outlined in their work are essential for developing energy-efficient materials and understanding lattice vibrations. This knowledge aids in creating thermoelectric materials and optimizing electronic devices, showcasing the practical impact of thermal physics in materials science and technology development.
6.2 Biological Systems and Medical Applications
Thermal physics plays a vital role in understanding biological systems, from heat transfer in living organisms to energy metabolism. Kittel and Kroemer’s principles are applied in studying airway hyperresponsiveness and muscle contraction models. The textbook’s insights into thermodynamic processes aid in developing medical technologies, such as thermal imaging and cancer treatment methods. These applications highlight the interdisciplinary relevance of thermal physics in advancing biomedical research and improving healthcare diagnostics and treatments.
6.3 Atmospheric and Environmental Science
Thermal physics is essential for understanding atmospheric processes, including energy transfer and thermodynamic principles governing weather patterns. Kittel and Kroemer’s concepts are applied in climate modeling to study heat distribution and phase transitions in the atmosphere. The textbook’s insights into statistical mechanics and phase transitions aid in analyzing environmental phenomena, such as airway hyperresponsiveness in stochastic models. These principles also inform strategies for sustainability, highlighting the role of thermal physics in addressing global environmental challenges and reducing emissions.
The Role of Kittel and Kroemer’s Textbook
Kittel and Kroemer’s Thermal Physics serves as a foundational textbook, bridging theory and practical applications. Its clear explanations and comprehensive coverage make it indispensable for both students and researchers, shaping understanding and innovation in the field.
7.1 Influence on Thermal Physics Education
Kittel and Kroemer’s Thermal Physics has profoundly shaped thermal physics education. Its clear, concise explanations and rigorous mathematical approach have set a standard for teaching the subject. The textbook’s ability to bridge theory and practical applications has made it a cornerstone in university curricula worldwide. By emphasizing fundamental concepts and their real-world relevance, it has inspired generations of physicists and engineers, fostering a deeper understanding of thermodynamic principles and their interdisciplinary applications. Its influence continues to grow, ensuring its place as a seminal educational resource.
7.2 Key Features of the Textbook
Kittel and Kroemer’s Thermal Physics is renowned for its clarity and depth. It provides a rigorous mathematical foundation, blending theoretical concepts with practical applications. The textbook’s structured approach ensures a logical progression from basic principles to advanced topics. Problem sets at the end of each chapter enhance understanding and application. Its emphasis on connecting thermodynamics with statistical mechanics makes it invaluable for graduate-level studies. The inclusion of modern research examples, such as band theory in liquid water, highlights its relevance to cutting-edge scientific inquiries.
7.3 Impact on Research and Development
Kittel and Kroemer’s Thermal Physics has significantly influenced research and development in various scientific domains. Its rigorous approach to thermodynamics and statistical mechanics has provided a foundation for advancements in materials science, solid-state physics, and biological systems. Researchers utilize its principles to study phase transitions, critical phenomena, and energy systems. The textbook’s emphasis on modern applications, such as band theory in liquid water, has inspired cutting-edge investigations in condensed matter physics and beyond, making it a cornerstone for both theoretical and experimental research.

Solving Problems in Thermal Physics
Kittel and Kroemer’s Thermal Physics equips students with robust problem-solving strategies, emphasizing analytical thinking and mathematical rigor. It addresses challenges in thermodynamics and statistical mechanics, providing clear methodologies for tackling complex scenarios, from energy systems to phase transitions, ensuring a deep understanding of thermal physics principles and their practical applications.
8.1 Common Challenges in Thermal Physics Problems
Solving thermal physics problems often involves complex systems and nonlinear interactions. Kittel and Kroemer’s Thermal Physics highlights challenges such as understanding phase transitions, managing non-equilibrium dynamics, and interpreting stochastic processes. Students frequently struggle with applying statistical mechanics to real-world scenarios and handling mathematical derivations of thermodynamic potentials. Additionally, visualizing energy distributions and connecting macroscopic observations to microscopic theories can be daunting. Mastering these challenges requires a strong foundation in both theoretical concepts and practical problem-solving techniques, as emphasized in the textbook.

8.2 Strategies for Effective Problem Solving
Mastering thermal physics problems requires a systematic approach. Start by identifying key concepts and simplifying complex systems. Use analogies from Kittel and Kroemer’s Thermal Physics to relate abstract ideas to familiar scenarios. Practice with worked examples to build intuition. Break problems into smaller parts, focusing on one variable at a time. Verify derivations step-by-step and cross-check results with physical intuition. Regular review of foundational theories, such as thermodynamic potentials and statistical mechanics, ensures a solid problem-solving foundation. This structured method enhances clarity and precision in tackling challenging problems.
8.3 Case Studies from Kittel and Kroemer
Kittel and Kroemer’s Thermal Physics includes insightful case studies that illustrate key concepts through real-world applications. These examples, such as phase transitions in solids and thermal properties of magnetic materials, demonstrate how theoretical principles solve practical problems. The textbook’s approach to analyzing heat capacity, lattice vibrations, and electron transport phenomena provides students with a clear understanding of complex systems. By focusing on both classical and quantum systems, these case studies bridge theory and experimentation, offering a comprehensive learning experience that enhances problem-solving skills and deepens conceptual understanding.
Modern Research in Thermal Physics
Modern research in thermal physics explores new frontiers, integrating Kittel and Kroemer’s Thermal Physics with interdisciplinary applications, advancing our understanding of complex systems and materials.
9.1 Current Trends and Advances
Current trends in thermal physics focus on quantum systems, nanotechnology, and biological applications. Advances in computational modeling and experimental techniques enable deeper insights into complex phenomena. Researchers are exploring non-equilibrium thermodynamics and quantum thermodynamics, bridging gaps between theory and application. These developments, influenced by Kittel and Kroemer’s foundational work, drive innovation in materials science, energy systems, and medical technologies, highlighting thermal physics’ evolving role in addressing modern scientific challenges.
9.2 Interdisciplinary Applications
Thermal physics principles, as outlined in Kittel and Kroemer’s work, are integral to diverse fields like materials science, biology, and environmental science. Advances in understanding phase transitions and energy transfer are applied to develop new materials and medical technologies. In biology, thermal physics aids in studying cellular processes and metabolic rates. Environmental science leverages these concepts to model climate systems and energy exchange. Such interdisciplinary applications highlight the versatility of thermal physics in addressing complex, real-world challenges across scientific domains.

9.3 Future Directions in Thermal Physics
Future research in thermal physics, inspired by Kittel and Kroemer’s foundational work, focuses on quantum systems and biological processes; Advances in band theory and RedOx potential studies, like those involving liquid water, offer new insights into energy transfer and phase transitions. These developments could revolutionize materials science, biomedicine, and environmental modeling. Emerging technologies, such as quantum computing and nanotechnology, also rely on thermal physics principles. Interdisciplinary collaborations will drive innovation, ensuring thermal physics remains central to addressing global challenges and advancing scientific understanding.
Kittel and Kroemer’s Thermal Physics provides foundational insights, essential for advancing materials science and quantum systems, ensuring its relevance in future scientific and educational endeavors.
10.1 Summary of Key Concepts
Kittel and Kroemer’s Thermal Physics provides a rigorous exploration of thermodynamic principles, statistical mechanics, and phase transitions. It emphasizes the laws of thermodynamics, entropy, and the role of probability in physical systems. The textbook bridges classical and quantum approaches, offering insights into thermodynamic potentials and their applications. Practical examples and mathematical derivations make it a cornerstone for both students and researchers, ensuring a deep understanding of thermal physics and its relevance in modern scientific advancements.
10.2 The Legacy of Kittel and Kroemer’s Work
Kittel and Kroemer’s Thermal Physics has left an indelible mark on the field, shaping modern understanding and education in thermal physics. Their work has inspired generations of physicists and engineers, providing a foundational text that bridges theory and application. The textbook’s clarity and depth have made it a benchmark for teaching and research, ensuring its enduring relevance in advancing scientific knowledge and technological innovation.
10.3 Final Thoughts on the Importance of Thermal Physics
Thermal physics remains a cornerstone of modern science, offering insights into energy, matter, and their interactions. Its principles underpin advancements in technology, materials science, and biological systems. The study of thermal processes continues to address global challenges, from energy efficiency to environmental sustainability. As research evolves, thermal physics adapts, ensuring its relevance in understanding complex phenomena and driving innovation across disciplines.